

To cytochemically demonstrate the presence of histones (basic proteins) in blood film of frog and human epithelial cheek cells using Fast Green FCF (at pH 8.1).
Protein solutions are amphoteric i.e. they can act as acids or bases depending upon the pH of the solution. A protein molecule at its isoelectric point has a net charge of zero due to the balance of positive and negative charges between the dissociated basic and acidic groups of the constituent amino acids. In this state of dissociation, the protein molecule exists as a zwitterion. The basic group having +ve charge in amino acid is mostly NH4+ group present in lysine, histidine and arginine. The acidic carboxyl groups of glutamic acid and acidic hydroxyl group of tyrosine and serine are primarily responsible for giving proteins their –ve charge.
The influence of pH on the dissociation of free basic and acidic groups of amino acids is shown by the addition of acid or base to a protein solution at its isoelectric point. Every protein has a specific isoelectric point known as pI. At this point + and – ions are equal. The total charge is 0. It is a general property of proteins that when they are exposed to pH which is below their pI then the amino groups of the protein dissociate i.e. it picks up a proton and becomes positively charged whereas if the proton is exposed to a pH above the pI then the carboxyl group dissociates losing a proton and the protein molecule become negatively charged.
In almost all organisms with the exception of viruses and bacteria, basic proteins histones occur. Their basic nature is due to the presence of lysine (amino acid) predominantly in histones and arginine predominantly in protamine. Cytochemical detection of these proteins can be done by taking the advantage of their basic side groups (-NH2) to bind with the acidic or cationic dyes such as fast green FCF.
The pI for histones is between pH 10-12. In the staining experiments on nuclear proteins (nuclear protein staining test), given by Alfert and Geschwind the staining is done at pH 8.1, which is below the pI for histones. So the histones become positively charged and bind with the negatively charged acid dye Fast green. Before staining, DNA has to be removed from the nucleohistone complex with hot 5% TCA as the phosphate groups of the nucleic acid block the basic amino groups of lysine and arginine. At pH 8.1, the staining is restricted to histones only.
Formaldehyde fixed material is used because it is a good fixative for proteins producing certain cross-linkages with the protein molecule so that the proteins are preserved in form similar to as they were in living conditions.
Following steps are involved in fixation of proteins by HCHO:
1. HCHO combines with the amino group of the protein forming an intermediate compound known as methylol.
2. Methylol further reacts with another amino group of a protein and releases a molecule of water and forms a crossing link or methylene bridge between two protein chains, therefore the proteins are chemically preserved within the cell.
Step 1: Smear Preparation
Human Cheek Epithelial Cells
Nuclei of Frog RBC and human cheek epithelial cells stain green because of presence of histones and cytoplasm remain colorless.
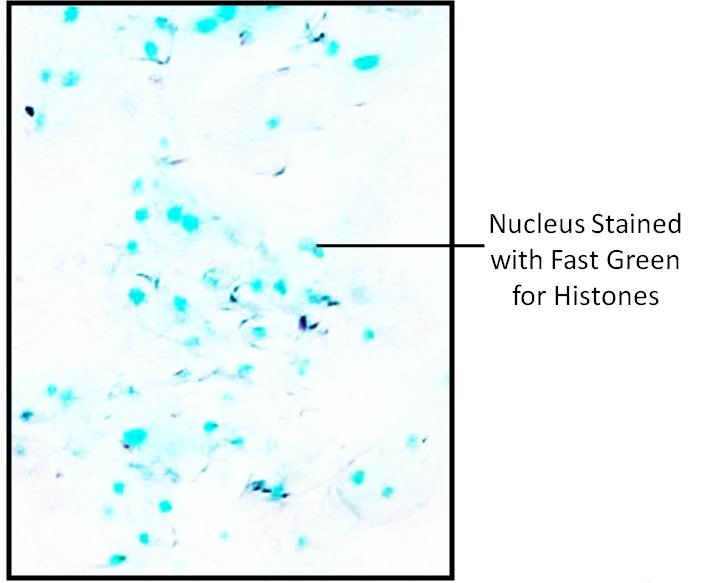 Fig.: Human Cheek Epithelial cells showing presence of Histones stained with Fast Green at pH 8.1
Fig.: Human Cheek Epithelial cells showing presence of Histones stained with Fast Green at pH 8.1
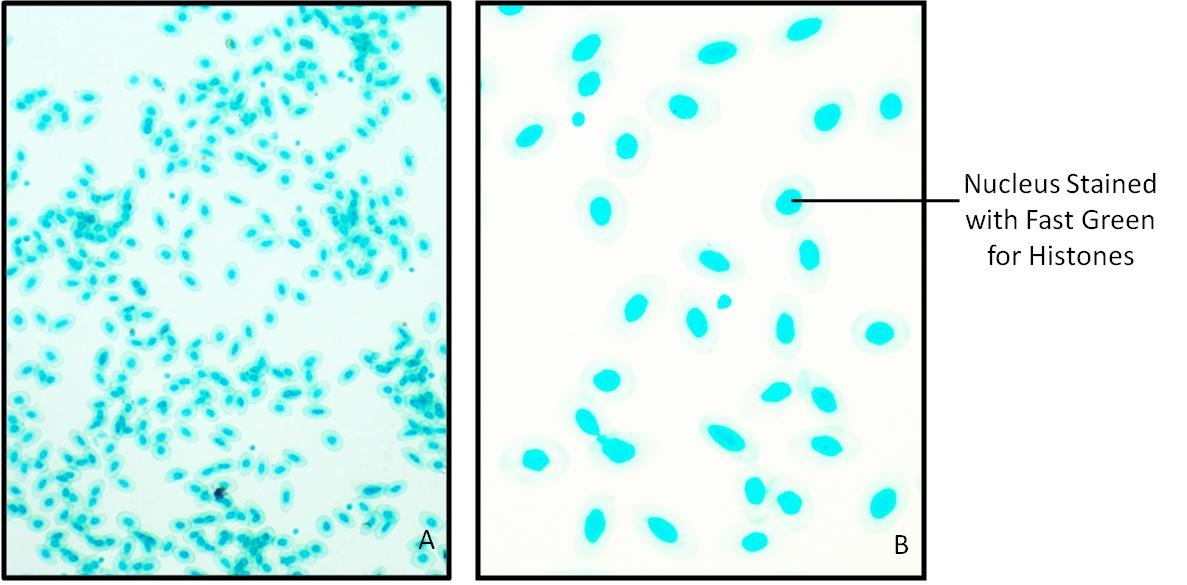
Fig.14.2: Frog RBC showing presence of Histones stained with Fast Green at pH 8.1.
A. 100 X B. 400 X
Precautions