

Quantification of DNA in unknown sample by diphenylamine (DPA) reagent.
All glassware and plastic ware used should be sterilized:
When DNA is treated with diphenylamine under the acidic condition a blue colored complex is formed which has an absorption peak at 595nm.This reaction is given by 2- deoxypentose in general. In acidic solution deoxypentose are converted into a highly reactive β hydroxyl leavulinic aldehyde which reacts with diphenylamine gives blue complex. In DNA, only the deoxyribose of purine nucleotide reacts so that the value obtained represents one half of the total deoxyribose produced.

Click here to perform the simulation

straight line graph is formed by taking concentration of standard DNA in ug/ml
along Xaxis and absorbance at 595nm along the Y-axis.
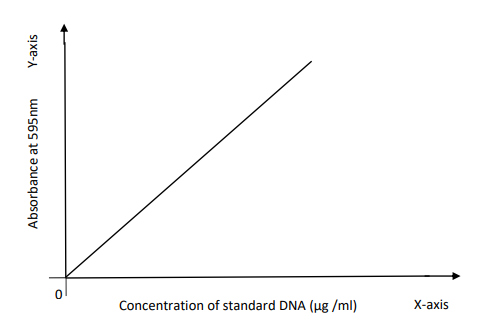
Slope = y2-y1/x2-x1
The amount of the DNA present in the given unknown solution = ________μg/ml.
Deoxyribose in presence of acid converts to a compound that binds with
diphenylamine to form a blue coloured complex. In standard DNA solution the
gradient of blue colour represents the corresponding DNA concentration.
Optical density of lowest concentrated DNA (100 βg/ml) is _____ and optical
density of highest concentrated DNA (1000 µg/ml) is______. The concentration
of unknown DNA is ___ βg/ml.